MediPharm Labs Reports Fourth Quarter and Full Year Results
Annual Revenue and Adjusted EBITDA Improve by 50%
TORONTO, March 27, 2024 /PRNewswire/ - MediPharm Labs Corp. (TSX: LABS) (OTCQB: MEDIF) (FSE: MLZ) ("MediPharm", "MediPharm Labs" or the "Company") a pharmaceutical company specialized in precision-based cannabinoids, today announced its financial results for the full year and three months ended December 31, 2023.
- 2023 full year revenue increased approximately 50% to $33M versus prior year of $22M.
- 2023 full year Adjusted EBITDA(1) improved 50% to negative $10M in 2023 from negative $21M in 2022.
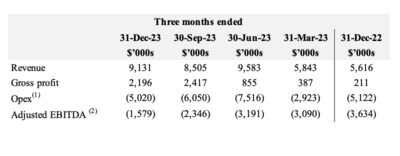
- Q4 2023 gross profit was $2.2M or 24%. Gross profit showed significant improvement over Q4 2022 of $0.2M or 4%.
- Q4 2023 Adjusted EBITDA(1) of negative $1.6M improved 55% from negative $3.6M in Q4 2022 and improved over 30% from negative $2.4M in Q3 2023. Adjusted EBITDA(1) continues to improve driven by margin expansion initiatives and cost reductions.
- Strong balance sheet, relative to many peer companies, with approximately $18M million of cash and less than $3 million of debt as of December 31, 2023.
- In Q4 2023, MediPharm completed an in-person inspection from the Brazilian Health Regulatory Agency (ANVISA). The first cannabis company in North America to complete an in-person inspection for Good Manufacturing Practice (GMP) cannabis production for Brazil.
- Brazil, with a population of 215M, has seen tremendous growth in medical cannabis patients with a 480% increase in prescriptions in 20221. MediPharm has two medical cannabis products with ANVISA authorizations and will increase deliveries to a new pharma partner in 2024.(2)
- In Q4 2023, MediPharm completed the development and initial deliveries of THC isolate, commonly known as Dronabinol, for the European Union market. Subsequent to year-end MediPharm has made deliveries of this product to multiple customers and it anticipates it having a materially positive impact to sales and gross profit. (2)
- In 2023, MediPharm expanded its top Australian medical cannabis flower brand, Beacon Medical, to include high quality GMP oils and vapes. Australia is the largest medical cannabis market outside of North America which The Pennington Institute (Cannabis in Australia 2023 report) estimates generated over $400m AUD in 2023.2
- MediPharm achieved a 2023 gross margin of 18% versus negative 9% in 2022. Positively moving to 24% in Q4 2023. This improvement is a trend the Company believes will continue. With the success in improving product mix and cost of goods sold MediPharm now has the foundation for a profitable future.
- Total Opex, which includes general administrative, marketing and selling, and research and development expenses, was reduced by $2M or 10% on a year over year basis, while growing sales by 50% in the same time period. Additional reductions implemented in Q4 2023 will further reduce Opex in 2024. (2)This execution of doing more with less has resulted in a revised cost base, positioning the company for profitability.
- MediPharm plans to further improve on profitability in 2024 including optimizing facility utilization with additional contract manufacturing, growing sales of healthy margin products, increasing international sales with local pharmaceutical partners, and refreshing the Canadian adult use and wellness portfolio to grow sales in our largest addressable market.
David Pidduck, CEO, MediPharm Labs commented, "MediPharm now has the margins, Opex and Adjusted EBITDA results all trending in the right direction. We also have a robust revenue pipeline with multiple partners in multiple markets. The transformation to a profitable growing company continues. Over the years we have invested in our infrastructure as a high quality and high capacity pharmaceutical grade manufacturer, allowing us to grow sales with new opportunities and markets, without additional investment into capital or resources. We are proud of the work completed in 2023 and excited about the future of MediPharm Labs."
Greg Hunter, CFO, MediPharm Labs added, "2023 was a transformation year for MediPharm, with the acquisition of VIVO, revenue increased by approximately 50% year over year and expanded our reach into Australia and the Canadian Medical business. Gross margins expanded significantly to 24% in Q4 and OPEX was reduced to produce the best Adjusted EBITDA in 4 years. In Q4, we continued to make progress by improving gross margins, reducing expenses and reducing cash burn as we drive towards profitability. Adjusted EBITDA of negative $1.6 million improved sequentially and year over year and is our best result in over 4 years. In addition, we ended the year with a strong balance sheet with $18 million of cash and less than $3 million of debt. MediPharm is in a strong financial position to capitalize on our strong suite of licences, global customer contracts and assets as we move in to 2024"
Financial Summary
(1) Opex includes general administrative expense, marketing and selling expenses and R&D expenses. |
(2) Adjusted EBITDA is a non-IFRS measure. See "Non-IFRS Measures". |
MediPharm's executive management team will also host a conference call and audio webcast on Wednesday, March 27, 2024 at 8:30 a.m. eastern time to discuss the Company's financial results.
Toll-free number: +1 (888) 330-2379 / International number: +1 (240) 789-2710
Conference ID: 4921762
Participants are asked to dial in approximately 15 minutes before the start of the call.
An audio webcast will be available by visiting the following link here.
For those who are unable to participate on the live conference call or webcast, a replay will be available at https://www.medipharmlabs.com/investors approximately one day after completion of the call.
Founded in 2015, MediPharm Labs specializes in the development and manufacture of purified, pharmaceutical-quality cannabis concentrates, active pharmaceutical ingredients (API) and advanced derivative products utilizing a Good Manufacturing Practices certified facility with ISO standard-built clean rooms. MediPharm Labs has invested in an expert, research driven team, state-of-the-art technology, downstream purification methodologies and purpose built facilities with five primary extraction lines for delivery of pure, trusted and precision-dosed cannabis products for its customers. Through its wholesale and white label platforms, MediPharm Labs formulates, develops (including through sensory testing), processes, packages and distributes cannabis extracts and advanced cannabinoid-based products to domestic and international markets.
In 2021, MediPharm Labs received a Pharmaceutical Drug Establishment Licence from Health Canada, becoming the only company in North America to hold a domestic Good Manufacturing Licence for the extraction of natural cannabinoids. The Company carries out its operations in compliance with all applicable laws in the countries in which it operates.
In 2023, MediPharm acquired VIVO Cannabis Inc. which expanded MediPharm's reach to medical patients in Canada via Canna Farms medical ecommerce platform, and in Australia and Germany through Beacon Medical PTY and Beacon Medical GMBH. This acquisition also included Harvest Medical Clinics in Canada which provides medical cannabis patients with Physician consultations for medical cannabis education and prescriptions.
- This is a non-IFRS reporting measure. See "Non-IFRS Measures" below.
- This is a forward-looking statement and based on a number of assumptions. See "Cautionary Note Regarding Forward-Looking Information" below.
This press release contains references to "Adjusted EBITDA", which is a non-IFRS financial measure. Management believes that this supplementary non-IFRS financial measure provides useful additional information related to the operating results of the Company. This non-IFRS financial measure is not recognized under IFRS and, accordingly, users are cautioned that this measure should not be construed as an alternative to net income (loss) and gross profit determined in accordance with IFRS as measures of profitability or as alternatives to the Company's IFRS-based Financial Statements. The non-IFRS measure presented may not be comparable to similar measures presented by other issuers. Adjusted EBITDA is a measure of the Company's overall financial performance and is used as an alternative to earnings or income in some circumstances. Adjusted EBITDA is essentially net income (loss) with interest, taxes, depreciation and amortization, non-cash adjustments and other unusual or non-recurring items added back. Adjusted EBITDA has limitations as an analytical tool as it does not include depreciation and amortization expense, interest income and expense, finance fees, gain in revaluation of derivative liabilities, taxes, government grants including rent and wage subsidies, one-off transactions, impairment losses on inventory and on fixed assets and intangibles, write down of deposits and share-based compensation. Because of these limitations, Adjusted EBITDA should not be considered as the sole measure of the Company's performance and should not be considered in isolation from, or as a substitute for, analysis of the Company's results as reported under IFRS. Adjusted EBITDA, as used within the Company's disclosure, may not be directly comparable to Adjusted EBITDA used by other reporting issuers. Adjusted EBITDA does not have a standardized meaning and the Company's method of calculating such non-IFRS measure may not be comparable to calculations used by other companies bearing the same description.
The following table reconciles the Company's net operating income (loss) (as reported) and Adjusted EBITDA for the periods presented:
Three months ended | Twelve months ended | |||
December 31, $'000s | December 31, $'000s | December 31, $'000s | December 31, $'000s | |
Net operating loss | (2,935) | (6,390) | (18,252) | (29,533) |
Adjusted for: | ||||
Share-based compensation expense | 306 | 1,390 | 2,027 | 2,872 |
Depreciation and amortization | 717 | 540 | 2,516 | 2,872 |
Restructuring related severance expenses | 335 | - | 2,303 | 1,233 |
Government grants | - | - | - | (21) |
Transaction fees | - | 813 | 883 | 1,164 |
Recovery of impaired receivables (1) | - | - | (2,010) | - |
Impairment loss on remeasurement of | - | - | - | - |
Impairment loss on remeasurement of assets | 23 | 13 | 40 | 81 |
Gain on disposition of assets | (174) | - | (174) | - |
Incremental cost of cannabis inventory | 372 | - | 2,427 | - |
Fair value adjustments in gross profit | (223) | - | (1,188) | - |
Write down of inventories (3) | - | - | 1,204 | 766 |
Adjusted EBITDA | (1,579) | (3,634) | (10,224) | (20,566) |
- This relates to the reversal of a former impairment of a long outstanding receivable.
- Incremental cost of cannabis inventory acquired in a business combination represents the fair value realized on sale of cannabis inventory acquired in a business combination.
- This adjustment is for unusual inventory write-downs only and not the total value of inventory written down.
This news release contains "forward-looking information" and "forward-looking statements" (collectively, "forward-looking statements") within the meaning of the applicable Canadian securities legislation. All statements, other than statements of historical fact, are forward-looking statements and are based on expectations, estimates and projections as at the date of this news release. Any statement that involves discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, assumptions, future events or performance (often but not always using phrases such as "expects", or "does not expect", "is expected", "anticipates" or "does not anticipate", "plans", "budget", "scheduled", "forecasts", "estimates", "believes" or "intends" or variations of such words and phrases or stating that certain actions, events or results "may" or "could", "would", "might" or "will" be taken to occur or be achieved) are not statements of historical fact and may be forward-looking statements. In this news release, forward-looking statements relate to, among other things, statements regarding: the Company's progress toward profitability; potential improvements in gross margin and revenue, potential future and annualized savings to be realized as a result of Company's restructuring efforts; growth of the Brazilian medical cannabis market, ability to gain further medical cannabis product approvals in Brazil; the Company's ability to increase deliveries to its Brazil partners in 2024; future German deliveries of THC isolate and the impact of such continued deliveries to the Company's sales and gross profit; Australian medical cannabis market size and growth potential; ability to optimize facility utilization; and ability to grow profitable sales. Forward-looking statements are necessarily based upon a number of estimates and assumptions that, while considered reasonable, are subject to known and unknown risks, uncertainties, and other factors which may cause the actual results and future events to differ materially from those expressed or implied by such forward-looking statements. Such factors include, but are not limited to: general business, economic, competitive, political and social uncertainties; the inability of MediPharm to obtain adequate financing; the delay or failure to receive regulatory approvals; and other factors discussed in MediPharm's filings, available on the SEDAR+ website at www.sedarplus.ca. There can be no assurance that such statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on the forward-looking statements and information contained in this news release. Except as required by law, MediPharm assumes no obligation to update the forward-looking statements of beliefs, opinions, projections, or other factors, should they change.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/medipharm-labs-reports-fourth-quarter-and-full-year-results-302100604.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/medipharm-labs-reports-fourth-quarter-and-full-year-results-302100604.html
SOURCE MediPharm Labs Corp.